Imbriano Carol research activity
- 1. NF-Y transcriptional and non-transcriptional control of cell proliferation
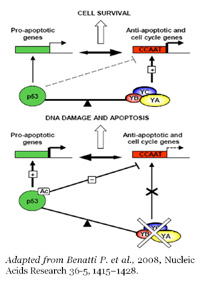
A biochemical and genetic link has been described between the transcription factor NF-Y and the oncosuppressor p53. NF-Y modulation, caused by inactivation and overexpression of the DNA-binding subunit of the complex (NF-YA), triggers cell cycle arrest and apoptosis, but the contribution of the p53-genetic background has been never carefully studied.
We demonstrated (Benatti P. et al., NAR 2011 and BBAGRM 2016) that NF-YA inactivation by shRNA leads to down-regulation of a multitude of cell cycle and metabolic genes, a delay in S-phase progression, DNA damage and apoptosis. The increase of the DNA damage marker γH2AX and PhosphoSer15-p53 in NF-YA inactivated cells supports the activation of a DNA-damage pathway. NF-YA loss induces the recruitment to DNA of the replication proteins PCNA, Mcm7 and of the intra-S checkpoint p53 variant, Δp53, consistently with a response to DNA replication defects.
The goal of our research will be to identify the role of NF-Y in transcriptional and non-transcriptional regulation of cell proliferation. In addition we aim to investigate the special partnership between NF-Y and wtp53 or mutp53 and to shed light on possible implications for cancer therapy. We are going to study in details the cellular, biochemical and gene expression response to NF-Y modulation, with respect to the status of p53.
Moreover, we are going to characterize the different activity played by NF-Y splice variants in controlling cell proliferation of cancer cells.
FUNDING:
# AIRC-IG 14210, "The NF-Y-p53 connection: implications on cancer cell survival and death".
# FONDAZIONE VIGNOLA- Pilot Sudy, "Identificazione dei meccanismi molecolari che controllano il soppressore tumorale p57kip2: basi per lo sviluppo di una nuova strategia terapeutica contro il tumore prostatico".
- 2. Identification of the role of transcription factor NF-Y and of its splice variants in myogenic differentiation and skeletal muscle regeneration
Skeletal muscle regeneration is supported by satellite cells (SCs), tissue-resident stem cells which contribute to regenerative myogenesis following muscle injury in postnatal skeletal muscle.
Several evidences suggest an involvement of the transcription factor NF-Y in SCs function. Indeed, one of the most active DNA motifs in genes upregulated in pluripotent cells is the CCAAT box, which is specifically bound by NF-Y. The DNA binding subunit of the complex, NF-YA, is encoded by two spliced transcripts (NF-YAs and NF-YAl), whose specific expression has been closely associated to stemness and proliferation rather than differentiation.
A role for NF-Y in the proliferation of skeletal muscle stem cells has been suggested in vivo: while NF-YA is absent in the myonuclei of wt mice, it is expressed in the nuclei of muscle fibers of mdx mice, animal models for Duchenne Muscular Dystrophy.
We aim to identify the function of NF-Y in SCs function in vivo. In particular, we intend to characterize the specific role of NF-YA splice variants in controlling the proliferation and self-renewal of SCs. Through genome-wide approaches we will be able to determine the NF-Y regulome during SCs activation. The activity of NF-Y during skeletal muscle regeneration will be studied and we hope that these data will pave the way to new NF-Y-mediated therapy to counteract muscle wasting caused by muscular genetic diseases.
FUNDING:
# AFM-Telethon Trampoline Grant 16408, "NF-YAs pharmacological therapy to potentiate the proliferative capacity of muscle satellite cells".
# AFM-Telethon, Investigator Grant 18364, "NF-YA as a molecular switch with therapeutic potential in muscle regeneration"